Consent forms#
A consent form is a document that provides potential participants with detailed information about the study, including its purpose, procedures, potential risks, benefits, and their rights as participants. It ensures that individuals fully understand the research and voluntarily agree to participate. By signing the form, participants acknowledge that they have been informed about the study and consent to their involvement.
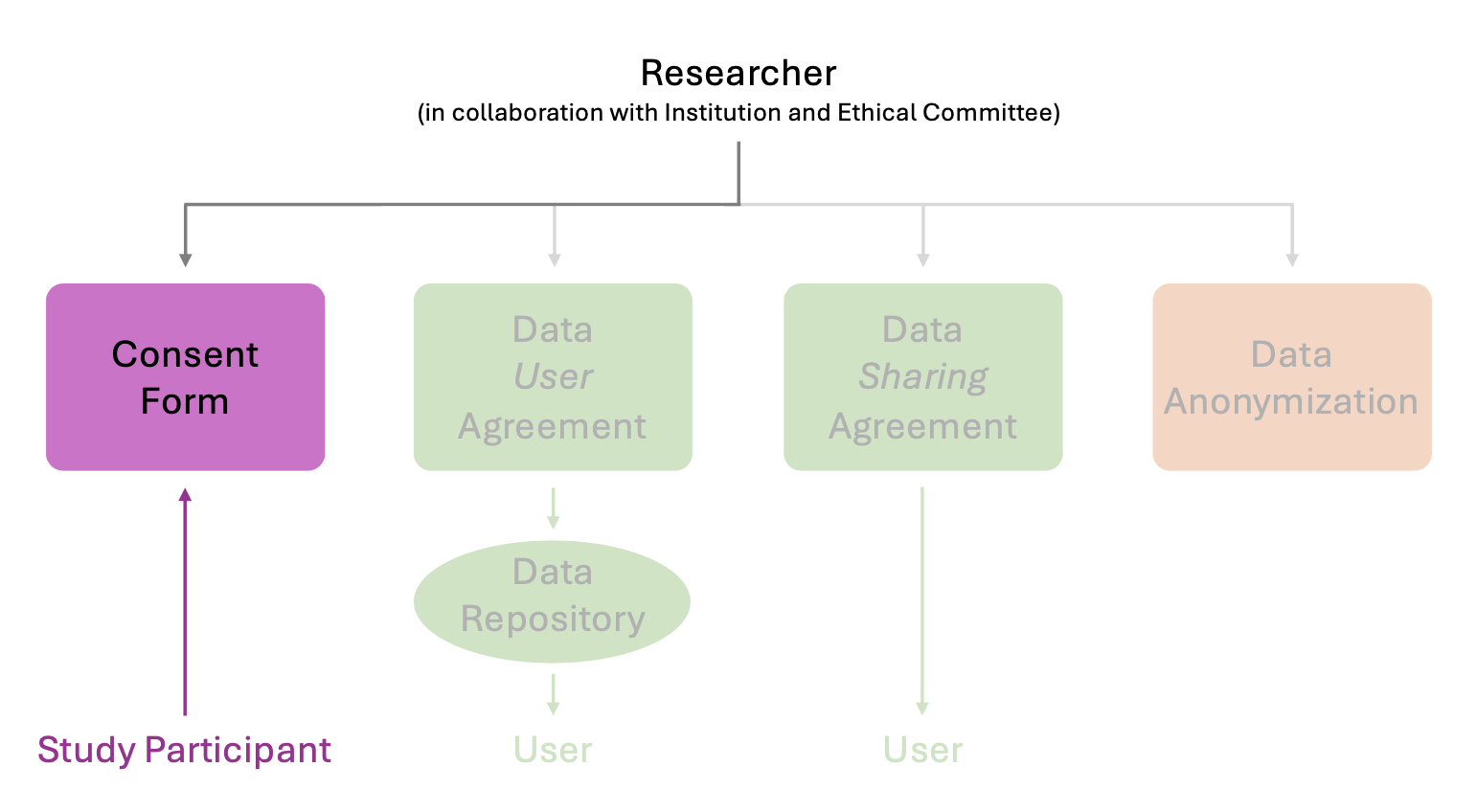
Templates of generic consent forms#
The two forms below are an adaptation of the Open Brain Consent [Bannier et al., 2021] to musculoskeletal imaging. You may choose to change the language to be restrictive depending on your own and institutional requirements. The Open Brain Consent kindly provides these documents in several languages. At this time we have text in English only.
The template of the Non-DGPR Consent Form is recommended for researchers working outside the European Union, whereas the template of the DGPR Consent Form is recommended for researchers working in the European Union.
Non-DGPR Consent Form (all data shared publicly)
(modified from Version: OBC-ULT 1.0.1 of the Ultimate Consent Form)
The data we share with the general public will not have your name on it, only a code number, so people will not know your name or which data are yours. In addition, we will not share any other information that we think might help people who know you guess which data are yours.
If you change your mind and withdraw your consent to participate in this study (you can call <PI name> at <phone number> to do this), we will not collect any additional data about you. We will delete your data if you withdraw before it was deposited in the database. However, any data and research results already shared with other investigators or the general public cannot be destroyed, withdrawn or recalled.
By agreeing to participate, you will be making a free and generous gift for research that might help others. It is possible that some of the research conducted using your information eventually could lead to the development of new methods for studying the Musculoskeletal System, new diagnostic tests, new drugs or other commercial products. Should this occur, there is no plan to provide you with any part of the profits generated from such products and you will not have any ownership rights in the products.
To the best of our knowledge, the data we release to the general public will not contain information that can directly identify you. The data will not have your name on it, only a code number, so people will not know your name or which data are yours. In addition, the data will not include data that we think might help people who know you guess which data are yours, such as your facial features or the date that you participated. If we write a report or article about this study or share the study data set with others, we will do so in such a way that you cannot be directly identified. However, by using additional data linked to your name (for example medical imaging scans obtained from your medical records) one could potentially associate your imaging or other information in our database back to you. In addition a security breach (break in or cyber attack) might lead to someone being able to link you to your data. This risk is very low because your data are stored in a secure database, and the information about your identity is stored separately from the data themselves, linked only through a code.
We will keep the private portion (name, contact information etc.) of your data in a secure location for at least <x> years. This way if one of the researchers that obtained the data from us will find something in your scans that would have a diagnostic value we will be able to contact you. After this period of time we will destroy this information to protect your privacy.
Letting us use and share your data is voluntary. However, you must be willing to share your data in this way in order to participate in this study. If you are not willing, you cannot participate in this study.
By signing below, you agree to provide your data for future research. You agree that these may be shared with other investigators at other institutions from around the world. The details, results, and implications of these studies are unknown.
DGPR Consent Form
(modified from Version: OBC-ULT 1.0.1 of the Ultimate Consent Form)
The current version of the ultimate consent form (GDPR edition) is given below. This contains GDPR-specific wording that relates to privacy implications due to the intention of processing and sharing the participant’s data. As such, this is seen as the Privacy Notice form. Informed consent to take part in the study is still recorded for the participant in the main consent form. The form includes some sentences in square brackets [ ], which indicates that they may be removed and/or replaced depending on your local legislation and ethics committee. Some sentences include fields, surrounded by <> to be entered.
Usage and storage of your data
While the collection, use and storage of your data are done for the purpose of conducting the study to which you are currently participating, these data might also be used for other future research projects in the field of musculoskeletal research. This includes data from your musculoskeletal system, and may also include test results from the study you took part in, family and medical history, and also data such as gender and age.
By agreeing to participate, you will be making a free and generous gift for research that might help others. [It is possible that some of the research conducted using your information eventually could lead to the development of new methods for studying the musculoskeletal system, new diagnostic tests, new drugs or other commercial products. Should this occur, there is no plan to provide you with any part of the profits generated from such products and you will not have any ownership rights in the products.] We ask for your consent to this access to your data.
Confidentiality of your data
To the best of our knowledge, the data we release will not contain information that can directly identify you using reasonable means. To protect your privacy, the data will be given a code, so people will not know your name or which data are yours. Your name, but also other information that can directly identify you, will be omitted. Data can only be traced back to you using information only available to the data processor (i.e. people involved in the study). This information will remain safely stored in the local research institute. The data cannot be traced back to you in reports and publications about the study. However, by using additional data linked to your name (for example medical imaging scans obtained from your medical records) one could potentially associate your imaging or other information in our database back to you. The risks of accessing such data from our servers have however been assessed and are considered to be low (see the university/centre Data Privacy Impact Assessment @<URL>).
Access to your data for verification
Some people can access all your data at the research location, including the original data with your name. This is necessary to check whether the study is being conducted in a good and reliable manner and to be able to notify you and your physician in case of any incidental findings resulting from your scans. Persons who have access to your data for review are: the local committee that monitors the safety of the study, the data controller
Retention period of your data
There is no plan to delete your data as they can be re-used for legitimate research interest. We will however re-evaluate every <number of years> years if it is worthwhile keeping them.
Withdrawing consent
You can withdraw your consent to the use of your personal data at any time. This applies to this study and also to the sharing for future research. You have however to understand that once shared with other institutions, it is impossible to remove your data from such copies.
Passing on to countries outside the European Union (EU)
Your encoded data can also be accessed by and sent to countries outside the EU. This is necessary so that non-EU based scientists can run analyses to verify the scientific results produced from this study or for future unrelated research. In those countries, the EU rules on the protection of your personal data do not apply. However, your privacy will be protected at an equal level, by means of a future link provided here Data User Agreement.
More information about your rights regarding processing of the data
For general information about your rights regarding the processing of your personal data, you can consult the website of the <Institution> Data Protection Authority.
If you have questions about your rights, please contact the person responsible for the processing of your personal data. For this study, that is:
<contact information> (see Appendix for contact details)
If you have questions or complaints about the processing of your personal data, we advise you to first contact the research location. You can also contact the Data Protection Officer of <Institution> (see the contact details in Appendix ) or the <Institution> Data Protection Authority.
Date:
Collected by:
Signature:
Examples of institutional consent forms#
Various institutions have different formulations for informed consent forms. Find below samples from some institutions of the members of the ORMIR community.
University of Calgary (Conjoint Health Research Ethics Board), Canada
Please note that this is not a “blanket” consent, but part of a study-specific consent form. If the participant agrees to use of the data in future research, a new ethics application would be required that describes the new study, and the source of data. However, we are not required to ask each participant to reconsent if they have already agreed via the text below.
Full consent form template: https://research.ucalgary.ca/conduct-research/ethics-compliance/human-research-ethics/conjoint-health-research-ethics-board-chreb
It is your choice whether or not to let researchers share your data and biospecimens for research in the future. If you say “yes,” you can change your mind later, but your data and biospecimens might still be used if they have already been shared.
My de-identified research data may be kept for use in future research to learn about, prevent or treat other health-related problems.
YES
NO
Hamilton Health Sciences, Ontario, Canada
Source: AMBERS Study
Schulthess Klinik, Zürich, Switzerland
Translated from German with DeepL. The original document in German can be downloaded here.
I authorize that my encrypted data from this research project may be used for medical research.
I understand that the data is encrypted and that the key will be stored securely. The data can be sent to other databases in Switzerland and abroad for analysis if they adhere to the same standards as in Switzerland. All legal data protection requirements are complied with.
I make my decision voluntarily and can withdraw this decision at any time. If I withdraw, my data will be anonymized. I only inform the project management and do not have to justify this decision.
Normally, all data is evaluated as a whole and the results are published in summary form. If there is an important result for my health, it is possible that I will be contacted. If I do not wish to be contacted, I will inform the project management.
If results from the data are commercialized, I am not entitled to a share of the commercial use.
UK Biobank
UK Biobank is a “large long-term biobank study in the United Kingdom which is investigating the respective contributions of genetic predisposition and environmental exposure to the development of disease”[Contributors, 2024].
The consent form for participants is composed of an information leaflet and the form itself. The section related to data reuse is the following:

National Institutes of Health (NIH)
Since January 2023, NIH requires Data Management and Sharing Plans as part of new applications for funding. Complete details are documented at https://sharing.nih.gov. General considerations and guidelines for languages used for informed consent are included in this following document and are worth a careful review.
We have adapted this language for MSK imaging applications, excluding language around biospecimens. If you plan to collect biospecimens as part of your study and need to include consent language to share access to biospecimens, then refer to the original language linked in the above document.
DESCRIPTION
This study is collecting data from you. We would like to make your data available for other research studies that may be done in the future. The research may be about similar diseases or conditions to this study. However, research could also be about unrelated diseases, conditions, or other types of research. These studies may be done by researchers at this institution or other institutions, including commercial entities. Our goal is to make more research possible. We plan to keep your data for [Insert time frame as indicated in the study protocol].
Your data may be shared with researchers around the world. However, the decision to share your data is controlled by [indicate which entity has control]. To get your data, future researchers must seek approval from* [indicate which entity has control]. The researchers must agree not to try to identify you.
Option #1: If the data are coded and can be linked back to the identity of the participant:
We will protect the confidentiality of your information to the extent possible. Your data will be coded to protect your identity before they are shared with other researchers. [indicate which entity has the code key] will have a code key that can be used to link to your identifying information. The code key will be securely stored.
Option #2: If the data cannot be easily linked back to the identity of the participant:
Your name and identifying information will be removed from any data you provide before they are shared with other researchers. Researchers cannot easily link your identifying information to the data.
CONSENT/WITHDRAWAL
Option 1: When sharing of data will be optional (e.g., for studies that have potential benefit):
It is your choice whether or not to let researchers share your data for research in the future. If you say “yes”, you can change your mind later. If you say “no”, you can still fully participate in this study.
If you change your mind and no longer wish to have us store or share your data, you should contact [insert contact info]. We will do our best to honor your request and to retrieve any data that have been shared with other researchers. However, there may be times we cannot. For example, if we do not have a way to identify your data we will not be able to retrieve them. In addition, if the data have already been used for new research, the information from that research may still be used.
Please initial [or sign depending on institutional practice] next to your choice:
______YES, use my data in other research studies
______NO, do NOT use my data in other research studies
Option 2: When sharing of data will not be optional (e.g., where sharing is integral to the purpose of the study):
Participating in this study means you agree to share your data. You can change your mind later, but researchers might still use your data if they have already been shared. If you do not want your data used for other projects, you should not participate in this study.
RISK / BENEFITS
[Risks] We will do our best to protect your data during storage and when they are shared. However, there remains a possibility that someone could identify you. There is also the possibility that unauthorized people might access your data. In either case, we cannot reduce the risk to zero.
[Benefits] You will not receive any direct benefit from sharing your data. However, sharing your data may contribute to research that could help others in the future.